Have you considered improving your medical diagnostic kits with cyclodextrins?
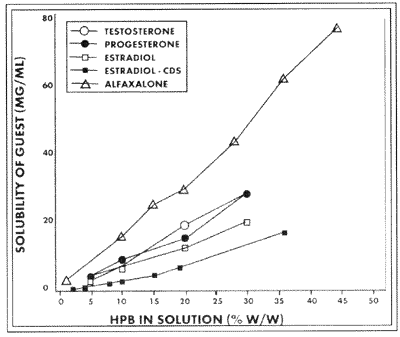
Hydroxypropyl Beta CyclodextrinCTD, Inc. has concentrated its efforts on the hydroxypropyl derivative of B-cyclodextrin (Trappsol® HPB), believing it to be the most likely candidate of all the chemically modified Cyclodextrins for incorporation into formulations used by humans and animals. HPBCD has the best balance of enhanced aqueous solubility, a wide range of drugs with which it forms stable complexes, and the most extensive collection of safety data in the technical literature with no adverse reactions reported. HPBCD is itself very soluble in water (greater than 500 mg/ml at room temperature compared to 18 mg/ml for B-Cyclodextrin) More importantly, HPB forms soluble complexes of the AL type [2] that is, the concentration of drug complexed increases linearly as the concentration of HPBCD increases [see Figure 3].
Figure 3: HPB is non-hygroscopic with inherently excellent tabletting properties. It does not change the surface tension of water as much as other Cyclodextrins such as dimethyl Beta-Cyclodextrin, which exerts a detergent-like effect on biological membranes. This property of dimethyl Beta-Cyclodextrin makes it much more hemolytic and irritating when administered intramuscularly or topically to mucous membranes.
When making aqueous solutions of HPBCD or HPBCD/drug complexes, a significant volume expansion must be accounted for. While the volume expansion is around 1.0 ml/gram at low (5 percent w/v) concentrations, the magnitude of the effect levels off at about 0.7 ml/gram at concentrations greater than 20 percent w/v. HPB is also moderately soluble in methanol and ethanol, providing opportunities for enhanced drug incorporation into powdered formulations using co-solvent techniques. The usual incorporation ratios of 10 or 20 to 1 (mg HPB required to complex 1 mg of drug) may be reduced to 7 or 8 to 1 by such solubilizing techniques. Aqueous solutions of THPB are stable to autoclaving temperatures and pressure effects; aqueous solutions of Trappsol HPBCD may be cold sterilized by filtration through a 0.2µ filter. In systemic, subacute and chronic toxicity studies, THPB was shown to be without significant clinical effect after IV dosing up to 200 mg/kg in either rats or monkeys. Single divided oral and IV doses of THPB as high as 15 g/kg and 10 g/kg respectively were not lethal to monkeys. Dr. Joseph Pitha of the NIH has experimentally evaluated many of the uses of this Cyclodextrin derivative and found that it can be conveniently applied to cell cultures and membrane preparations [14]. He also observed that THPB is non-toxic after oral, IP and IV administration to different rodent species. Solutions of THPB were not irritating to the eyes of rabbits, to human skin, or when injected into the brains of test animals. The maximum human dose of THPB given parenterally was approximately 500 mg/kg iv given continuously as a 5 percent aqueous solution to one individual for four days for the treatment of an endogenous vitamin metabolism disorder [15]; no adverse clinical effects were reported during those four days and none have been reported upon continued observation to this date. Most drug development groups have experienced the frustration of not being able to continue the development of certain novel compounds with promising biological activities because the active agent is either too unstable or too insoluble in water. A poorly water soluble compound may have inadequate or highly variable absorption when taken orally. When given by injection, therapeutic doses of such compounds may have to be given in unacceptably large volumes. They may also involve formulations requiring pH adjustments and/or organic solvents (alcohol, Tween®, castor oil, and so on) that result in painful or necrosing solutions for injection or infusion. The active agent itself may cause irritation at the injection site. These side effects may be ameliorated by Cyclodextrins. Compounds with inadequate solubility may have a short shelf life or require unusual storage conditions. Products produced by biotechnological processes commonly face problems of instability in water and/or loss of biological activity due to formation of aggregates or dimers, or by non-specific adsorption to container walls. These instabilities, formidable in new chemical entities, have been corrected in existing products by formulating with excipients that produce known side effects. Cyclodextrins offer new formulations that eliminate the previous compromises, thereby eliminating or significantly reducing unnecessary side effects. In addition to the parenteral and oral routes of administration, THPB complexes may be used in nasal sprays, transdermal preparations, and vaginal and rectal suppositories. The actual form of the formulation may be altered through the use of complexed material: for example, an emulsion can become a solution; an oily material may be turned into a powder. Undesirable characteristics such as smell or taste may in some cases be eliminated or improved. Storage of the Cyclodextrin/drug complex can often be made more convenient since, once a complex is formed, the solvent can be removed by lyophilization; and the resultant powder may be tabletted or stored at room temperature. Reconstitution in water at neutral pH can be done in seconds, if needed. The instability of certain drugs presents a major challenge to the formulation scientist. Cyclodextrins offer a novel approach to protecting chemically unstable drugs from reacting with their environment since formation of drug/cyclodextrin inclusion complexes often has a marked stabilizing and protective effect. Examples of improved drug stability are shown in Figures 5 and 6.
Figure 5:
Figure 6: Both an unstable anticancer drug (melphalan) and a chemically unstable dihydropyridine-linked drug (estradiol) show marked improvement in their stability under accelerated testing conditions. The extent to which a compound is stabilized must be determined empirically since it requires the formation of an inclusion complex and ultimately an understanding of which portion of the drug molecule fits into the cavity of the cyclodextrin, and whether that portion includes the reactive portion of the drug causing the instability. Since the usefulness of Cyclodextrins vary according to molecular weight, cavity size, intrinsic solubility in water and other solvents, safety, and cost, the formulation scientist must match the intended use of the drug with the appropriate Cyclodextrin or Cyclodextrin derivative. |
||||||||||||||||||||||||