Have you considered improving your medical diagnostic kits with cyclodextrins?
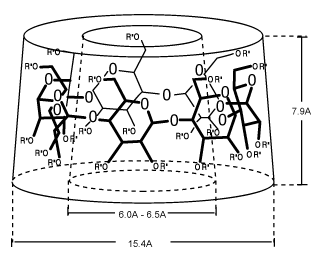
Natural CyclodextrinsCyclodextrins (CD's) as they are known today, were called cellulosine when first described by Villiers [1] in 1891. Soon after, Schardinger identified the three naturally occurring Cyclodextrins--alpha, beta, and gamma. These new compounds were referred to as Schardinger sugars. For 25 years between 1911 and 1935, Pringsheim in Germany was the leading researcher in this area, demonstrating that these sugars formed stable aqueous complexes with many other chemicals. By the mid 1970's, each of the natural cyclodextrins had been structurally and chemically characterized and many more complexes had been studied. Briefly, the natural Cyclodextrins are produced from starch by the action of cyclodextrin glycosyltransferase (CGTase), an enzyme produced by several organisms, Bacillus macerans being the earliest source. Structurally, Cyclodextrins consist of 6, 7, or 8 (
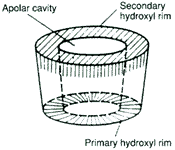
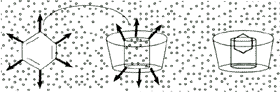
It is the interplay of atomic (Van der Waals), thermodynamic (hydrogen bonding), and solvent (hydrophobic) forces that accounts for the stable complexes that may be formed with chemical substances while in the apolar environment of the Cyclodextrin cavity. The complex exists in an equilibrium dependent upon the concentrations of the Cyclodextrin, the guest chemical and water. The rate at which the associated complex is formed is determined in large part by the accessibility of the guest molecule to the Cyclodextrin cavity and the magnitude of the net thermodynamic driving force.
Accessibility may be quantified as a statistical factor determined by the molecular geometry of the guest molecule and the particle size achieved. Complexes with drugs usually form quite rapidly (equilibrium may be attained in minutes) because even the most lipophilic compounds are solvated by water to some extent and these discrete hydrated molecular particles are better able to get past the hydrophilic hydroxyl groups at the entrance to the Cyclodextrin cavity. The hydrating molecules of water can actively interact with the hydroxyl groups on the rims of the Cyclodextrin toroid or they may simply shield the hydrophobic drug molecule from being repelled by the hydroxyl groups. Once past the rim hydroxyls, the hydrating molecules of water are driven from the hydrophobic cavity leaving the naked drug molecule to find its most stable resting place. In the case of extremely water insoluble substances, the true equilibrium may not be achieved for hours or days because molecular segregation due to hydration occurs extremely slowly. Once the molecule has entered the cavity, the "goodness of fit", as determined by the weak interactions taking place in the cavity, will make the final contribution to the association component of the equilibrium process. These weak forces can create selective interactions similar to those of enzymes.
Dissociation is usually an equally rapid process, most often caused by the sudden rapid increase in the number of water molecules outside the cavity. Even though there may be an initial energy barrier to dissociation, the concentration gradient created becomes overwhelming and the chemical is displaced. Unable to find relatively scarce Cyclodextrin molecules to reform the complex, the chemical exists free in solution or precipitates, depending on the amount of dilution and concentration of Cyclodextrin.
Stability constants of 10-100 are usual. A mathematical treatment of the association and dissociation constants of Cyclodextrins and chemical substances is elegantly discussed by Higuchi and Connors [2]. Stability constants of 10-100 are usual. Consideration of the characteristics of these interesting molecules leads to the conclusion that their use with drugs would be desirable in order to:
I.S. Pagington [3] and A. Parrish [4] give brief reviews of the general applicability of Cyclodextrins with examples. Dr. Josef Szejtli [5] provides a most comprehensive and up-to-date discussion of Cyclodextrins as a technology applicable to chemicals employed by many industries. With so much potential, why have the pharmaceutical applications of Cyclodextrins been so slow in achieving commercial significance in the hundred years scientists have known about them? The answers are cost, availability, false assessment of toxicity and inadequate aqueous solubility of the most widely studied Cyclodextrin, Beta-Cyclodextrin. It was not until the late 1970's that researchers started to chemically modify Cyclodextrins with the intention of solving the toxicity and aqueous solubility problems. In 1981, Josef Pitha [6] looked at the enhanced solubilizing effects on the fat soluble vitamins achieved by modifying each of the natural Cyclodextrins with alkyl groups. Since that time, Cyclodextrins have been modified with many different groups; technical papers describing complexes with chemically modified Cyclodextrins have proliferated and thousands of US patents have been issued. [7-9] |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||